Introduction
"Soil fungi contribute to a variety of ecosystem functions such as decomposition of organic matter, nitrogen and phosphorus cycling, and carbon storage (Treseder and Lennon 2015). Climate change has the potential to affect these functions by changing fungal community composition through warming (Geml et al. 2016; Treseder et al. 2016; Oliverio, Bradford and Fierer 2017) and more variable precipitation regimes (Hawkes and Keitt 2015; Averill, Waring and Hawkes 2016; Keitt et al. 2016). This in turn could alter rates of plant litter decomposition, carbon sequestration, and nitrogen mineralization, amongst other integral processes (Treseder 2016; Aamir et al. 2019; Cavicchioli et al. 2019). Therefore, understanding specifically how climate variation alters fungal community composition is critical to understanding whole ecosystem function in the face of climate change.
While changes in climate can impact fungal community composition (Classen et al. 2015; Andrew et al. 2016; Peay et al. 2017), the direction and strength of these effects are variable across systems (Rillig, Treseder and Allen 2002; Compant, Van Der Heijden and Sessitsch 2010) especially when considering the different effects on functional groups (i.e. saprotrophs, symbiotrophs, and pathotrophs) and species guilds (e.g. ectomycorrhizal fungi, arbuscular mycorrhizal fungi) (Mohan et al. 2014; Geml et al. 2015; Asemaninejad et al. 2018). However, trait-based approaches may reveal unifying patterns across these apparently discordant responses because they allow us to group taxa by function and therefore link fungal identity to mechanistic responses (Aguilar-Trigueros et al. 2014, 2015; Crowther et al. 2014; Koide, Fernandez and Malcolm 2014; Morgado et al. 2015; Rillig et al. 2015). By incorporating a trait-based approach to fungal community ecology, we can address a fundamental question: will fungal communities continue to provide the same functions under the stress of climate change (Pickles et al. 2012)? By linking climate-driven shifts in fungal community composition with functional traits, we can begin to connect changes in species diversity with ecosystem function.
Of fungal functional traits, modes of nutrient acquisition – in particular for species engaging in mutualisms with plant hosts – are especially crucial for understanding the effects of environmental filtering on ecosystem function. Symbiotrophs are mutualist fungi that associate with plant hosts, and mycorrhizae are a subset of this group that specialize in associations with plant roots in which the plant exchanges carbon resources for the mycorrhiza’s soil-derived nitrogen and phosphorus (Smith and Read 2008). Mycorrhizae play a major role in ecosystem function, as they provide almost half of a tree’s organic nitrogen budget (Zhang et al. 2019) and contribute the bulk of new carbon into soil (Zhang et al. 2018). Additionally, mycorrhizal groups (i.e. arbuscular mycorrhizal and ectomycorrhizal fungi) differ in the amount of carbon they input to soil (Averill, Turner and Finzi 2014) and their respective nitrogen and phosphorus benefits to their hosts (Teste, Jones and Dickie 2019). Therefore, understanding changes in mycorrhizal communities in particular can provide insight into changing ecosystem functions in the future (van der Heijden et al. 2015)."
Given that mycorrhizas are host-associated and therefore rely on a mutualism to fulfill their metabolic demands, there is potential for mutualisms to withstand climate-driven changes in fungal community composition. Most generally we would expect that, when comparing the microbial community of a single host species to the community at large, host-associated communities are more similar to each other than to the regional pool of species, which includes mutualist species (Peiffer et al. 2013; Lebeis 2015; Adair and Douglas 2017); taking this in the context of climate change, plant host association has the potential to dampen the effects of climate on community turnover, as fungal associates are more similar across differing climatic conditions within a single host species than the entire species pool (Nuccio et al. 2016). For mutualist species (i.e. mycorrhizal partners) specifically, species in these functional groups may be even more similar across sites than the whole host-associated community.
If mutualists are indeed buffered from climate impacts on composition by their hosts, this could imply the conservation of host function given future climate change because their mutualists will retain their own function. While mycorrhizal partners may remain similar in composition, changes in relative abundance of species functional traits could conserve host function (Eduardo et al. 2018; Yan et al. 2018). Thus, by filtering membership of the fungal community (Kiers et al. 2003), host trees may maintain mycorrhizal mutualism function across drastically different climates, thereby preserving ecosystem functions provided by their mycorrhizae. Therefore, understanding how to preserve host and mutualist function remains crucial for management and conservation strategies concerning woodland systems to adapt to climate change.
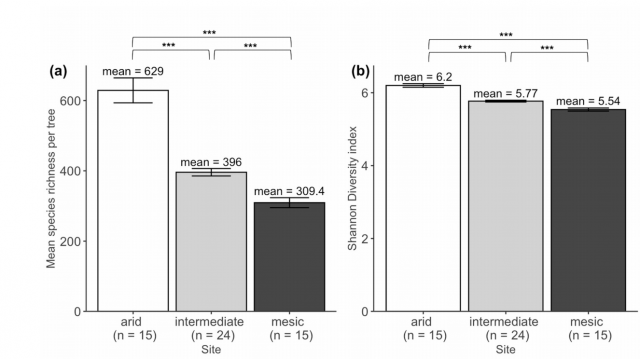
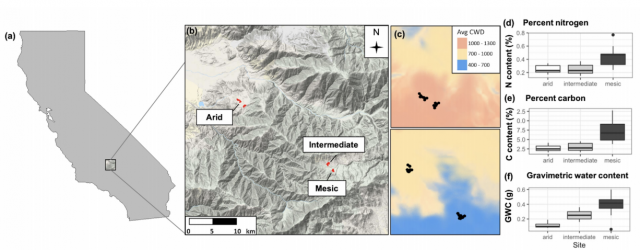
In this work, we use three climatically distinct sites to 1) quantify changes in fungal community composition, 2) test the effects of host association on these shifts, and 3) measure turnover in relative abundance of ectomycorrhizal species functional traits. An observational approach like the one taken here provides a spatial advantage to small-scale experiments by letting community assembly occur over a landscape (Bennett, Kasel and Tibbits 2009). Additionally, oak woodland systems in California are likely to experience range contractions and northward shifts as a result of changing temperatures and precipitation (Kueppers et al. 2005); therefore, they are representative of woodlands in Mediterranean climates globally, which are likely to experience range contractions as a result of increasing temperature, drought, and more variable precipitation (IPCC 2014). However, little is known about whether fungal associates can withstand or ameliorate climate change effects. Our site – the Tejon Ranch in the Tehachapi Mountains of California – is particularly ideal to test questions about climate effects on host-associated soil fungal communities because some hosts are constant across climatic conditions and there are distinct shifts in climate within a geographically constrained area (8 km) (McCullough et al. 2016). We first ask how fungal community composition responds to climate when holding host identity constant, and hypothesize that both richness and diversity will be highest at cooler, wetter sites than hot, dry sites (Peay et al. 2017). Across climatic conditions, soils in dry sites tend to be nutrient-poor compared to wet sites (Talmon, Sternberg and Grünzweig 2011; Delgado-Baquerizo et al. 2013; Jiao et al. 2016); therefore, arid environments will likely support fewer fungal species than their mesic counterparts (Maestre et al. 2015). Next, we ask how mycorrhizal association may drive convergence of fungal communities, and hypothesize that communities of mycorrhizal species are more similar across sites than whole host-associated communities. Potential fungal partners are likely filtered by their hosts based on efficacy of nutrient acquisition given carbon costs (Dickie 2007; Vályi et al. 2016; Powell and Rillig 2018), and we expect host selectivity to override the filtering effects of climate across sites. Lastly, we ask how functional trait composition of fungal mutualists reflects climate conditions in our sites, and hypothesize that functional traits will reflect the environment in which the species are found. Because ectomycorrhizal fungi provide nutrients and water to hosts (Tedersoo, May and Smith 2010), we anticipated that differences in composition in this community might reflect divergent tree nutritional needs (Moeller, Peay and Fukami 2014). In this case, we explore the functional traits of ectomycorrhizal fungi and predict that functional traits considered adaptive for stressful environments, in particular rhizomorph formation and foraging type, will be found in higher abundance in arid compared to mesic sites (Moeller, Peay and Fukami 2014)."
Citation
A Bui, D Orr, M Lepori-Bui, K Konecki, HS Young, HV Moeller. (2020) Soil fungal community composition and functional similarity shift across distinct climatic conditions. FEMS Microbiology Ecology: fiaa193